Minimum Inhibitory Concentration Testing Services
As an expert in providing solutions to support virology and microbiology research, Creative Diagnostics has recently launched a series of new Minimum Inhibitory Concentration (MIC) assay services to assist researchers in determining the lowest concentration of an antimicrobial drug required to inhibit the growth of a microorganism. This information is essential to ensure that antibiotics are effectively screened to increase the success rate of treatment.
The first step in antimicrobial discovery is usually to screen a library of drug candidates based on the MIC values of the target bacteria. As such, the MIC is often the starting point for further preclinical evaluation of new antimicrobials. The concept of MIC was originally developed by microbiologist Alexander Fleming, who used the turbidity of broth to evaluate the antimicrobial activity of antibiotics, which is generally considered to be the concept of MIC.
In the late 1980s, the Clinical and Laboratory Standards Institute (CLSI) consolidated the MIC assay methods and standards for clinical use to evaluate the bacteriostatic effect of antibiotics. In microbiology, the MIC is the lowest concentration of a chemical (usually a drug) that prevents the apparent growth of a bacterium. The MIC depends on the microorganism, the infected person (only in the body), and the antibiotic itself.
Combining infectious disease and analytical expertise with a commitment to providing clients with the most robust portfolio of in vitro antiviral and antimicrobial testing services, Creative Diagnostics now offers researchers a range of MIC testing services, including culturing of standardized test organisms to be tested, testing for minimum inhibitory concentration, and statistical analysis of results. Creative Diagnostics’ efficient, high-quality MIC testing services are competitively priced in the marketplace, and its team ensures 24/7 online service and timely feedback of results.
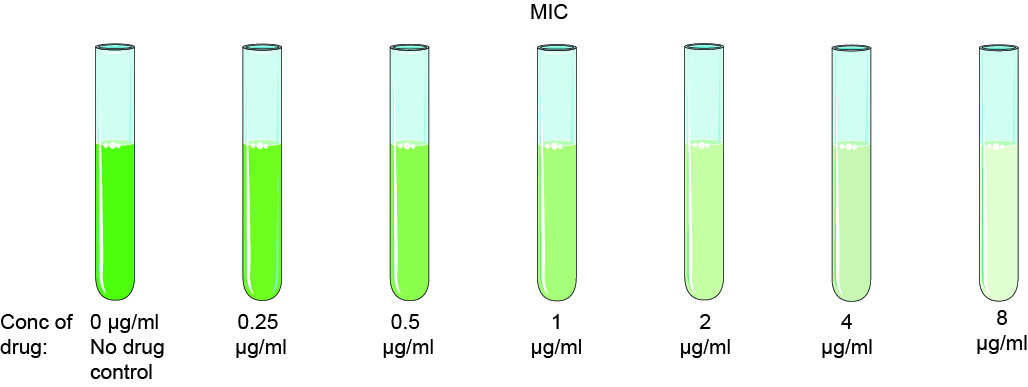
At Creative Diagnostics, MIC is determined by preparing test chemical solutions in vitro at increasing concentrations. The solution is first incubated with different batches of cultured bacteria. The results are then measured using either the agar dilution method or the broth microdilution method. Using agreed breakpoint values, the results are classified as susceptible (often referred to as sensitive), moderate or resistant to specific antibacterial agents.
Measurements are typically performed on planktonic (free-floating) bacteria, and the MIC can be determined from the optical density measured using a spectrophotometer or disk diffusion method. This is a time and cost saving strategy for screening and identifying promising drug candidates. The assay can also be adapted to determine the lowest test concentration (MIC50 and MIC90) required to inhibit the growth of a group of test isolates, i.e., 50% or 90%.
Creative Diagnostics works with scientists in industry and academia to support the early stages of antibacterial, vaccine, and diagnostic discovery and to advance the study of bacterial infections. The company can also develop custom assays based on customers’ specific processes. For more information, please visit https://antiviral.creative-diagnostics.com/minimum-inhibitory-concentration.html.
About Creative Diagnostics
Headquartered in New York, Creative Diagnostics is a consulting and experimental service provider specializing in virology and microbiology. The company provides comprehensive solutions to conquer obstacles in virology and microbiology research, from high-security infrastructure provision, biosafety regulation elucidation, to expert viral system assistance.


