Food and drug administration authorizes the initial at-household check for COVID and the flu : NPR
[ad_1]
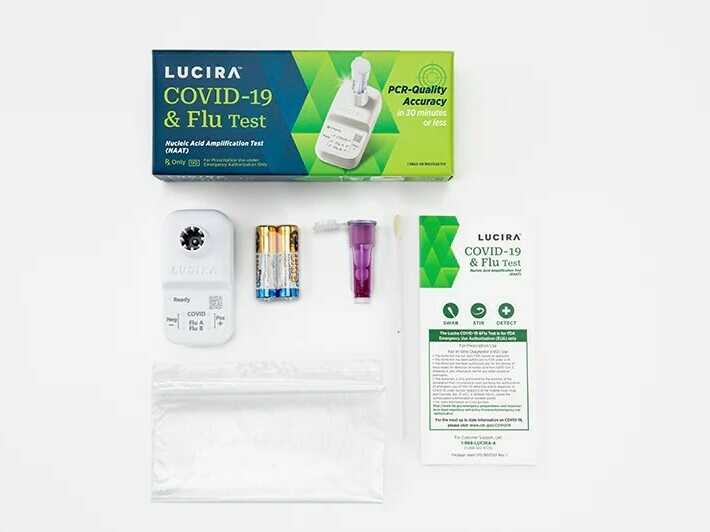
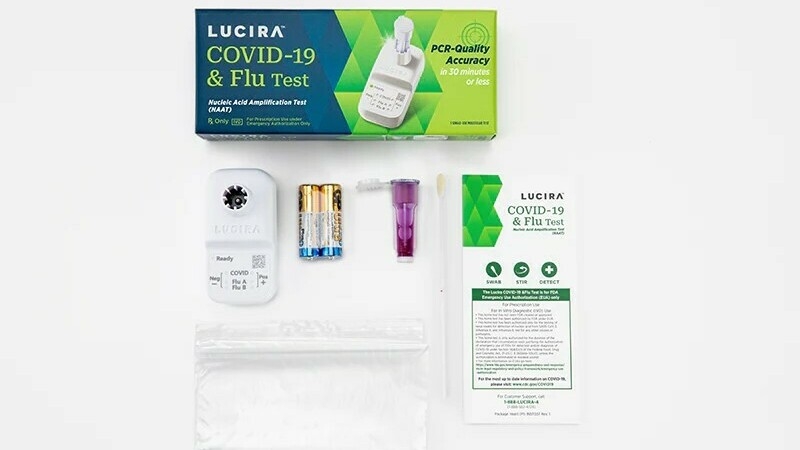
Lucira Wellness, which developed the twin-purposed diagnostic examination, said it can offer a beneficial result as quick as 11 minutes and a unfavorable end result in about 30 minutes.
Lucira Health
conceal caption
toggle caption
Lucira Health and fitness
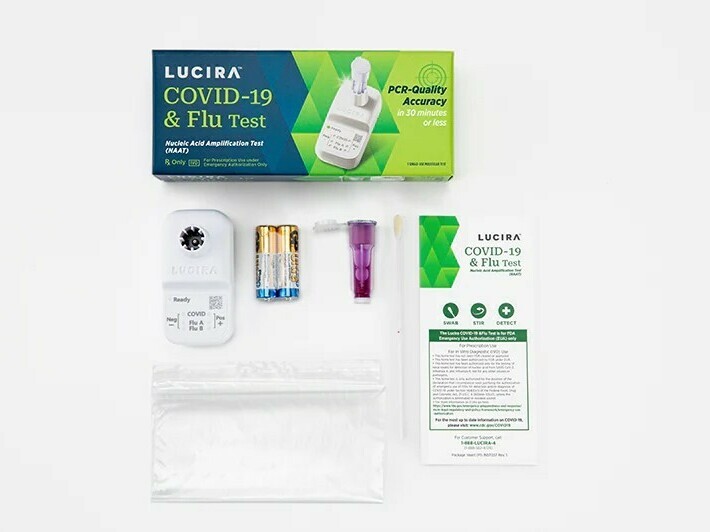
Lucira Wellness, which formulated the twin-purposed diagnostic examination, claimed it can provide a favourable result as rapid as 11 minutes and a detrimental outcome in about 30 minutes.
Lucira Well being
The Foods and Drug Administration issued an unexpected emergency use authorization on Friday for the first at-property test that can at the same time detect equally COVID-19 and the flu.
With a shallow nasal swab, the single-use kit can supply benefits within 30 minutes indicating irrespective of whether a individual is good or detrimental for COVID, as well as influenza A and influenza B, which are two common strains of the flu.
People 14 and older can frequently execute the take a look at on them selves, the Fda suggests. People in between the ages of 2 and 13 can get outcomes with the help of an grownup.
Dr. Jeff Shuren, the director of the FDA’s Heart for Products and Radiological Health and fitness, called the examination as a “significant milestone.”
“We are eager to carry on advancing higher accessibility to at-property infectious disease tests to most effective aid community health and fitness requirements,” Shuren mentioned in a assertion.
The exam was designed by Lucira Health, a California-centered corporation that was also the first to acquire Fda acceptance for at-home rapid COVID exams again in 2020.
According to the Fda, in people exhibiting indicators, the Lucira home kit correctly detected 88.3% of COVID infections and 90.1% of influenza A infections. The test can establish influenza B in lab experiments, the Food and drug administration mentioned. But mainly because there are not ample cases of the virus circulating in actual-world configurations, even more testing will be essential, officers reported.
The Fda also warned that, equivalent to all immediate diagnostic checks, there is a threat of phony good and false detrimental success. The agency states men and women who check favourable for COVID or the flu must consider correct safeguards and stick to-up with a wellness treatment company, while folks who receive a damaging outcome of either COVID or influenza B ought to ensure it with a molecular examination preformed in a lab.
Individuals who exam unfavorable but go on to experience indications of fever, cough or shortness of breath really should also adhere to up with their overall health care service provider in scenario of other respiratory viruses, the Fda claimed.
The dual-purposed test arrives right after a surge of COVID, the flu and respiratory syncytial virus — or RSV — that strained hospitals throughout the country previous slide.
“The collective effect of COVID-19, flu and RSV underscore the value of diagnostic exams for respiratory viruses,” the Food and drug administration reported in a assertion.
In excess of the earlier handful of months, COVID-related fatalities and hospitalizations have started to drop, in accordance to the latest info from the Centers for Sickness Handle and Prevention. Likewise, fees of flu and RSV-related hospitalizations have been going down, the CDC uncovered.
[ad_2]
Supply hyperlink





